Last two entries covered fairly dark areas in the history of drug development and testing. Today we will be looking at much more benign substances with much more benign histories- the butyl tryptamines. This post is very long. I'm splitting it into 2 parts. You can read part 2 here.
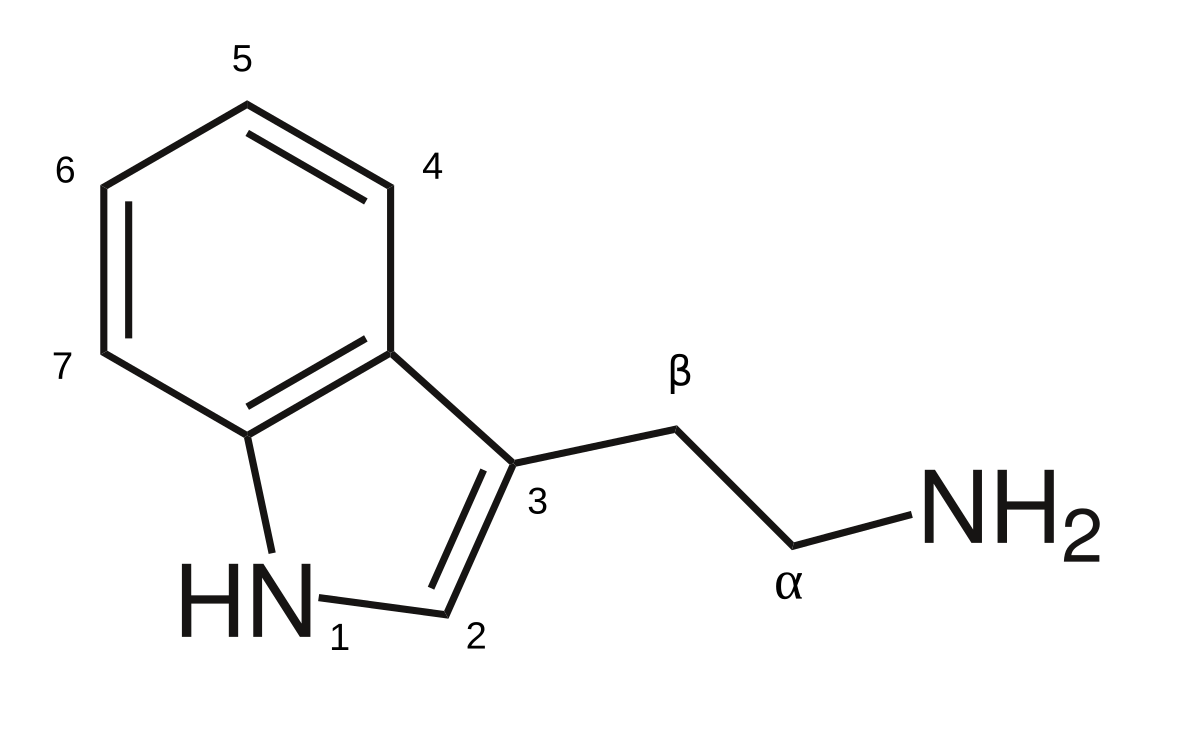 |
| The basic tryptamine structure |
To those familiar with tryptamines, you may be aware of the base structure of the tryptamine (if you're already familiar you can skip this section)- the indole (This is a phenyl (hexagon) ring connected to a pyrrole (pentagon) ring- if you're unfamiliar with organic chemistry you can just look the terms up it's fairly straightforward!), with an ethylamino (2 carbons and a nitrogen with the corresponding hydrogens filling space, labeled as NH2 on the diagram) attached to the pyrrole. The psychedelic tryptamines are constructed by attaching carbons to that nitrogen out on the end of that ethyl carbon chain. Say you attach 2 methyl (single carbon) groups, then you have DiMethylTryptamine, or DMT. If you remember the progression of carbon groups, it goes Methyl (1), Ethyl (2), Propyl (3) and Butyl (4). And we have seen various transmutations and combinations of most of those extensions on the market, with various other substitutions (Not to mention once you have >3 carbons you can rearrange them so they aren't just straight chain! This can yield Isopropyl groups, which are Y shaped, offering even more variation). Methylethyltryptamine (MET), Diethyltrytamine (DET), Methylpropyltryitamine (MPT), Ethylpropyltryptamine (EPT) and Dipropyltryptamine (DPT) (+ a number of isopropyl variants) have become the basis for a number of popular research chemicals, with new variations on those themes being developed and produced every year. So the logical next step is adding yet another carbon, forming a Butyl group.
 |
| The many ways you can arrange 4 carbons. I don't know why sec-butyl is highlighted, this was the only good picture I could find and I didn't feel like making a diagram myself |
This offers us such specimens as Dibutyltryptamine, Methylbutyltryptamine, Ethylbutyltryptamine, Propylbutyltryptamine, and then, oh god butyl groups can also be arranged into isobutyl and sec-butyl and tert-butyl forms (And the not pictured square shaped ring cyclobutyl form), expanding possible combinations by a whole other factor and a whole new mouthful of chemical nomenclature (Can you say Isobutylisopropyltryptamine?)! Yikes. An absurd magnitude of possible permutations aside, what are these chemicals like? Why haven't we seen them for sale anywhere, or seen any interest or curiosity in them? The case for most that have been attempted is lackluster but definitely present activity it seems. I will be looking at some of the variations that have been attempted and forgotten throughout history, along with speculating about some that have never been tried in humans or ever manufactured at all in human history.
Those that have a history of human usage come almost exclusively from the man, the myth, the legend, Alexander Shulgin, as documented in his seminal work TiHKAL (Tryptamines I have Known and Loved). I want to avoid delving too much into substances only explored in his works, because frankly there is nothing I could say on them that he hasn't already said better than I ever could. So I want to use that as a basis to look into some other related substances! The compounds Shulgin produced and tested which I will be looking at for part 1- MBT, DBT, NsBT, NtBT, and 4-HO-DBT. Part 2 of this post will look into the documentation on some other variations- NBT, 4-HO-DiBT, and 4-HO-DsBT, and from there, I will look at the vast field of other possibilities...
I am well aware of Shulgin's assessment that "Butyl is futile" as a dismissal of Butyl compounds as simply being so high dosing and lacking in interesting effects that they perhaps are not worth pursuing. While I certainly trust his judgment on that, I decided to still write about them at length because they are still an interesting variation on the theme of tryptamine substitution, lend some interesting observations with respect to structure-activity relations, and prevent a vast field of possibilities for various combinations of substitutions, many of which have never been attempted and may yield unexpected interesting effects.
MBT
The simplest butylated tryptamine explored by Shulgin was MBT, Methylbutyltryptamine. This sees a single methyl group and a looooong butyl group attached to the nitrogen.
Shulgin observed some properties in this one that I think set an important benchmark for all butylated tryptamines- Most importantly the dosage. You can go read the full entry in TiHKAL yourself, but with respect to doses he observes:
"(with 130 mg, orally) "Perhaps a subtle intoxication at two hours, and certainly nothing at five hours."
(with 175 mg, orally) "Some mild incoordination and concentration difficulties, all trivial, and a good sleep and a good day the next day."
(with 250 mg, orally) "At 75 minutes there was the prompt development of an intoxicated state primarily characterized by fine motor impairment. Nothing remotely resembling any type of hallucination. Appetite was normal and food and water were consumed without difficulty. Most activities were uninteresting, even dull. The effects lasted about five hours."
(with 400 mg, orally) "It hit an just over an hour, and it quickly became difficult to keep both eyes focused on the point of gaze. There was no actual double vision, but things were not quite right. In a few more minutes an apparent motion became apparent with fixed objects, and shortly thereafter there was a faint 'retinal circus' that was reminiscent of DMT but less compelling. Subject matter could not be chose, but rather came on its own. At this point, walking required great concentration, and lying on a bed was a much better choice. Music seemed to encourage the drifting of thoughts, but all the eyes-closed effects faded quite quickly. I felt overheated, sweat a lot, was intensely dehydrated, and drank quantities of water all night, and still felt dehydrated. Urine output was low. Not my choice of drug; the intoxication is too much for the visual stuff."
Those that have a history of human usage come almost exclusively from the man, the myth, the legend, Alexander Shulgin, as documented in his seminal work TiHKAL (Tryptamines I have Known and Loved). I want to avoid delving too much into substances only explored in his works, because frankly there is nothing I could say on them that he hasn't already said better than I ever could. So I want to use that as a basis to look into some other related substances! The compounds Shulgin produced and tested which I will be looking at for part 1- MBT, DBT, NsBT, NtBT, and 4-HO-DBT. Part 2 of this post will look into the documentation on some other variations- NBT, 4-HO-DiBT, and 4-HO-DsBT, and from there, I will look at the vast field of other possibilities...
I am well aware of Shulgin's assessment that "Butyl is futile" as a dismissal of Butyl compounds as simply being so high dosing and lacking in interesting effects that they perhaps are not worth pursuing. While I certainly trust his judgment on that, I decided to still write about them at length because they are still an interesting variation on the theme of tryptamine substitution, lend some interesting observations with respect to structure-activity relations, and prevent a vast field of possibilities for various combinations of substitutions, many of which have never been attempted and may yield unexpected interesting effects.
MBT
 |
| MBT |
Shulgin observed some properties in this one that I think set an important benchmark for all butylated tryptamines- Most importantly the dosage. You can go read the full entry in TiHKAL yourself, but with respect to doses he observes:
"(with 130 mg, orally) "Perhaps a subtle intoxication at two hours, and certainly nothing at five hours."
(with 175 mg, orally) "Some mild incoordination and concentration difficulties, all trivial, and a good sleep and a good day the next day."
(with 250 mg, orally) "At 75 minutes there was the prompt development of an intoxicated state primarily characterized by fine motor impairment. Nothing remotely resembling any type of hallucination. Appetite was normal and food and water were consumed without difficulty. Most activities were uninteresting, even dull. The effects lasted about five hours."
(with 400 mg, orally) "It hit an just over an hour, and it quickly became difficult to keep both eyes focused on the point of gaze. There was no actual double vision, but things were not quite right. In a few more minutes an apparent motion became apparent with fixed objects, and shortly thereafter there was a faint 'retinal circus' that was reminiscent of DMT but less compelling. Subject matter could not be chose, but rather came on its own. At this point, walking required great concentration, and lying on a bed was a much better choice. Music seemed to encourage the drifting of thoughts, but all the eyes-closed effects faded quite quickly. I felt overheated, sweat a lot, was intensely dehydrated, and drank quantities of water all night, and still felt dehydrated. Urine output was low. Not my choice of drug; the intoxication is too much for the visual stuff."
"
So this indicates that the effects are frankly mild and a bit uninteresting, but definitely there. There are notable uncomfortable physical and incapacitating effects. The psychedelic effects at the highest dose seem just barely past threshold even as physical effects increase. The overall duration given is 4-6 hours.
What's most noteworthy is the extremely high dosage of this drug- other unsubstituted tryptamines (MET, DET, DPT, MiPT, DiPT) yield mild experiences at doses of around 20-100 mg. Dosing any of them at 400 mg would be an overwhelming all-consuming experience, while for this one it seems lacking and gentle. Shulgin notes that it might be worthwhile to attempt different routes of administration for this one such as vaporization or IV (or perhaps insufflation!), or to combine it with an MAOI like P. harmala.
DBT
No this is not Dialectical behavioral therapy, but Dibutyltrytamine, or rather if you took the tryptamine molecule and gave it some gnarly horns. Shulgin manufactured this one, and mentioned that it showed a lower potency than DMT or DET when injected intramuscularly at 1 mg/kg. He at the very least doesn't note that it's completely inactive. He says there are further details under his entry for DMT, but this was a dead end. So the quality and precise effects of the DBT experience are still mostly a mystery.
Some other researchers however, delved into the possibilities of DBT in animal trials. A very recent study looked into 4 very odd substituted tryptamines, including DBT5. This study looked into the Head Twitch Response in mice- this is a diagnostic test to determine if a drug is active as a 5-HT2A agonist, the mechanism of action responsible for psychedelic effects in humans. Essentially, if the specific HTR is observed, it means that that compound is highly likely (though not certainly) to be hallucinogenic in humans, and that that mouse is highly likely (though not certainly) having a little mouse trip.
The results of this study were fairly analogous with the response demonstrated by known hallucinogenic tryptamines, indicating that DBT has a very high likelihood of being hallucinogenic in humans. So this definitely shows promise!
Another study by Brimblecombe et al from 1964 also looked at animal responses to a variety of tryptamines6- something interesting that this study noted was that there seemed to be a definite maximum dose of the more complex (more carbons stuck to the nitrogen) tryptamines-It seemed that it worked as a negative feedback loop, with a high enough concentration of the molecule preventing its own metabolism. This is an effect not observed in other psychedelics, and means that if a high enough dose is taken, no effects may be felt at all before the drug is excreted, unmetabolized. In rats, this dose was 50 mg/kg, which would be a dose in the range of multiple grams for humans. While this seems like a horrendous overdose of any psychedelic, it wasn't shown to be toxic in rats.
It is most likely however, that DBT would dose very high relative to other tryptamines. This study showed an active dose in rats was around 5 mg/kg, which would be a range of 300-400 mg in a human. Dose may also be inferred from structure- The current active psychedelic with the most carbons and hydrogens attached to the ethyl-amino chain is DPT, with 6 carbons and 14 hydrogens sticking out. This is also the highest orally dosing base tryptamine, with a common dose in the range of 150-250 mg. Take a step down to DET (4 carbons, 10 hydrogens). Thus it should follow that DBT, with 8 carbons and 18 hydrogens should dose higher. There doesn't seem to necessarily be an exact linear progression to this pattern so a precise dose wouldn't be known, but it would most likely be at least >100 mg for light effects. As with any base tryptamine, it would probably have a fairly short duration, from 5-6 hours at the longest.
NsBT & NtBT
Some new naming conventions come into play. The N- denotes that a simple hydrogen is stuck to the nitrogen in lieu of the usual carbon structure. The -sB- and -tB- denote the sec-butyl and tert-butyl variants of the 4-carbon butyl design, respectively, as there are many ways to rearrange 4 carbons.
What's most noteworthy is the extremely high dosage of this drug- other unsubstituted tryptamines (MET, DET, DPT, MiPT, DiPT) yield mild experiences at doses of around 20-100 mg. Dosing any of them at 400 mg would be an overwhelming all-consuming experience, while for this one it seems lacking and gentle. Shulgin notes that it might be worthwhile to attempt different routes of administration for this one such as vaporization or IV (or perhaps insufflation!), or to combine it with an MAOI like P. harmala.
DBT
 |
| DBT |
No this is not Dialectical behavioral therapy, but Dibutyltrytamine, or rather if you took the tryptamine molecule and gave it some gnarly horns. Shulgin manufactured this one, and mentioned that it showed a lower potency than DMT or DET when injected intramuscularly at 1 mg/kg. He at the very least doesn't note that it's completely inactive. He says there are further details under his entry for DMT, but this was a dead end. So the quality and precise effects of the DBT experience are still mostly a mystery.
Some other researchers however, delved into the possibilities of DBT in animal trials. A very recent study looked into 4 very odd substituted tryptamines, including DBT5. This study looked into the Head Twitch Response in mice- this is a diagnostic test to determine if a drug is active as a 5-HT2A agonist, the mechanism of action responsible for psychedelic effects in humans. Essentially, if the specific HTR is observed, it means that that compound is highly likely (though not certainly) to be hallucinogenic in humans, and that that mouse is highly likely (though not certainly) having a little mouse trip.
The results of this study were fairly analogous with the response demonstrated by known hallucinogenic tryptamines, indicating that DBT has a very high likelihood of being hallucinogenic in humans. So this definitely shows promise!
Another study by Brimblecombe et al from 1964 also looked at animal responses to a variety of tryptamines6- something interesting that this study noted was that there seemed to be a definite maximum dose of the more complex (more carbons stuck to the nitrogen) tryptamines-It seemed that it worked as a negative feedback loop, with a high enough concentration of the molecule preventing its own metabolism. This is an effect not observed in other psychedelics, and means that if a high enough dose is taken, no effects may be felt at all before the drug is excreted, unmetabolized. In rats, this dose was 50 mg/kg, which would be a dose in the range of multiple grams for humans. While this seems like a horrendous overdose of any psychedelic, it wasn't shown to be toxic in rats.
It is most likely however, that DBT would dose very high relative to other tryptamines. This study showed an active dose in rats was around 5 mg/kg, which would be a range of 300-400 mg in a human. Dose may also be inferred from structure- The current active psychedelic with the most carbons and hydrogens attached to the ethyl-amino chain is DPT, with 6 carbons and 14 hydrogens sticking out. This is also the highest orally dosing base tryptamine, with a common dose in the range of 150-250 mg. Take a step down to DET (4 carbons, 10 hydrogens). Thus it should follow that DBT, with 8 carbons and 18 hydrogens should dose higher. There doesn't seem to necessarily be an exact linear progression to this pattern so a precise dose wouldn't be known, but it would most likely be at least >100 mg for light effects. As with any base tryptamine, it would probably have a fairly short duration, from 5-6 hours at the longest.
NsBT & NtBT
 |
| NsBT (left) and NtBT (right) |
Some new naming conventions come into play. The N- denotes that a simple hydrogen is stuck to the nitrogen in lieu of the usual carbon structure. The -sB- and -tB- denote the sec-butyl and tert-butyl variants of the 4-carbon butyl design, respectively, as there are many ways to rearrange 4 carbons.
Both are mentioned in passing by Shulgin, lacking their own entries, in the commentary on another drug, NET (N-ethyltryptamine).
On NsBT he states:
"...the hydrochloride [salt melts] at ... 175-177 °C ... Interestingly, NSBT is one of the two mono-substituted tryptamines that just might have CNS activity. It has shown a generalized and somewhat diffuse intoxication with several studies covering the 25 to 75 milligram range. Short lived, intellectual excitement with some modest sensory enhancements. Promising, and a lot of erotic horniness, but no plus threes, yet."
While on NtBT He states:
"In the 5 to 20 milligram area, there is a light-headed intoxication that is a totally pleasant buzz, but nothing more profound than that. Wouldn't it be fascination of it turned out that all of the mono-tryptamines (the NRT's) were GHB-like intoxicants, and totally devoid of psychedelic activity. That would be a true challenge to the SAR crowd. I was told many years ago that NTBT was extremely potent when smoked, but I never received any particulars, and I must leave that as a baseless rumor."
This demonstrates both may be interesting stimulants, intoxicants, aphrodisiacs fitting the bill for drugs defined as Empathogens. They would be interesting to explore further. No other information exists beyond that.
4-HO-DBT
Now we get into substitutions- for those who don't know a substitution is where you take the base tryptamine molecule as defined by what's stuck to that nitrogen (xxT) and add things to other parts of it. Most tryptamines show effects with either an -OH or -AcO group stuck to the 4 position, or an -MeO on the 5 position (see the first image on this article). These substitutions usually increase potency and duration of the tryptamine and yield all variety of unique experiences.
So what can we expect from 4-HO-DBT? A tryptamine with greater oral activity, potency, and a longer duration than DBT. Shulgin made this one too, but curiously only tried it once at 20 mg, a dose where he observed no activity. He then tossed this one aside. I find this curious, because the butylated tryptamines have a clear pattern of being significantly less potent than other tryptamines, yet he didn't even bother to try a higher dose than is standard for most other smaller 4-HO tryptamines. But he was also a very busy man with a lot of drugs to test so I don't hold it against him.
The wikipedia article for this substance interestingly enough has an uncited claim that this was sold on the rc market and a few anecdotal reports note that it is indeed active, if a bit uninspiring at doses well past 20 mg (though it doesn't specify doses). I could not dig up any of these reports however. A cursory search did find this substance in the stock of some dubious surfaceweb vendors that I have never heard of whom I'm not sure I trust, but even if they're fake listings they are likely imitating a time it was on the market, because why else list this extremely obscure substance?
If you have by chance encountered this substance, please contact me and share your knowledge. Some amount of people out there have tried this and frustratingly none of them saw fit to share any of what they've learned with the rest of us. If you are consuming a drug for which there is 0 information available online, I believe you are OBLIGATED to share whatever information you have gained from your experiences with the rest of the community, whether it be descriptions of the experience or at the very least information on dosage and duration.
Part 2 coming after the jump.... It will look beyond Shulgin at some of the other butyl tryptamines that have been attempted, and then into the future of infinite possibilities...
You can continue if you'd like.
Sources and Further Reading:
1-Shulgin A, Shulgin A (1997) MBT. TiHKAL: The Continuation
2-Shulgin A, Shulgin A (1997) DBT. TiHKAL: The Continuation
3-Shulgin A, Shulgin A (1997) NET. TiHKAL: The Continuation
4-Shulgin A, Shulgin A (1997) 4-HO-DBT. TiHKAL: The Continuation
5-Abiero A, Ryu IS, Botanas CJ, et al. (2019) Four Novel Synthetic Tryptamine Analogs Induce Head-Twitch Responses and Increase 5-HTR2a in the Prefrontal Cortex in Mice. Biomol Ther (Seoul) 28(1):83‐91
6-Brimblecombe RW, Downing DF, Green DM, Hunt RR (1964) Some pharmacological effects of a series of tryptamine derivatives. Br J Pharmacol Chemother 23(1): 43–54.
7-Leonard BE, Shallice SA (1972) The effects of some tryptamine derivatives on brain monoamines and their precursor amino acids. Neuropharmacology 11(3):373‐384.
4-HO-DBT
 |
| 4-HO-DBT |
So what can we expect from 4-HO-DBT? A tryptamine with greater oral activity, potency, and a longer duration than DBT. Shulgin made this one too, but curiously only tried it once at 20 mg, a dose where he observed no activity. He then tossed this one aside. I find this curious, because the butylated tryptamines have a clear pattern of being significantly less potent than other tryptamines, yet he didn't even bother to try a higher dose than is standard for most other smaller 4-HO tryptamines. But he was also a very busy man with a lot of drugs to test so I don't hold it against him.
The wikipedia article for this substance interestingly enough has an uncited claim that this was sold on the rc market and a few anecdotal reports note that it is indeed active, if a bit uninspiring at doses well past 20 mg (though it doesn't specify doses). I could not dig up any of these reports however. A cursory search did find this substance in the stock of some dubious surfaceweb vendors that I have never heard of whom I'm not sure I trust, but even if they're fake listings they are likely imitating a time it was on the market, because why else list this extremely obscure substance?
If you have by chance encountered this substance, please contact me and share your knowledge. Some amount of people out there have tried this and frustratingly none of them saw fit to share any of what they've learned with the rest of us. If you are consuming a drug for which there is 0 information available online, I believe you are OBLIGATED to share whatever information you have gained from your experiences with the rest of the community, whether it be descriptions of the experience or at the very least information on dosage and duration.
Part 2 coming after the jump.... It will look beyond Shulgin at some of the other butyl tryptamines that have been attempted, and then into the future of infinite possibilities...
You can continue if you'd like.
Sources and Further Reading:
1-Shulgin A, Shulgin A (1997) MBT. TiHKAL: The Continuation
2-Shulgin A, Shulgin A (1997) DBT. TiHKAL: The Continuation
3-Shulgin A, Shulgin A (1997) NET. TiHKAL: The Continuation
4-Shulgin A, Shulgin A (1997) 4-HO-DBT. TiHKAL: The Continuation
5-Abiero A, Ryu IS, Botanas CJ, et al. (2019) Four Novel Synthetic Tryptamine Analogs Induce Head-Twitch Responses and Increase 5-HTR2a in the Prefrontal Cortex in Mice. Biomol Ther (Seoul) 28(1):83‐91
6-Brimblecombe RW, Downing DF, Green DM, Hunt RR (1964) Some pharmacological effects of a series of tryptamine derivatives. Br J Pharmacol Chemother 23(1): 43–54.
7-Leonard BE, Shallice SA (1972) The effects of some tryptamine derivatives on brain monoamines and their precursor amino acids. Neuropharmacology 11(3):373‐384.

Revolade 25mg Tablet is used to treat low platelet count due to chronic immune thrombocytopenic purpura or chronic hepatitis C virus (HCV) infection. It is also used to treat severe aplastic anemia. It works by stimulating the formation of new platelets in the blood. Platelets help to reduce or prevent bleeding.Take this medicine in the dose and duration as per doctors prescription. mandatory to take advice from doctor.
ReplyDeleteThis is such an informative post! This Blog really caught my attention. The information you've shared here are really helpful.
ReplyDeleteFind the information about revolade 25mg here.