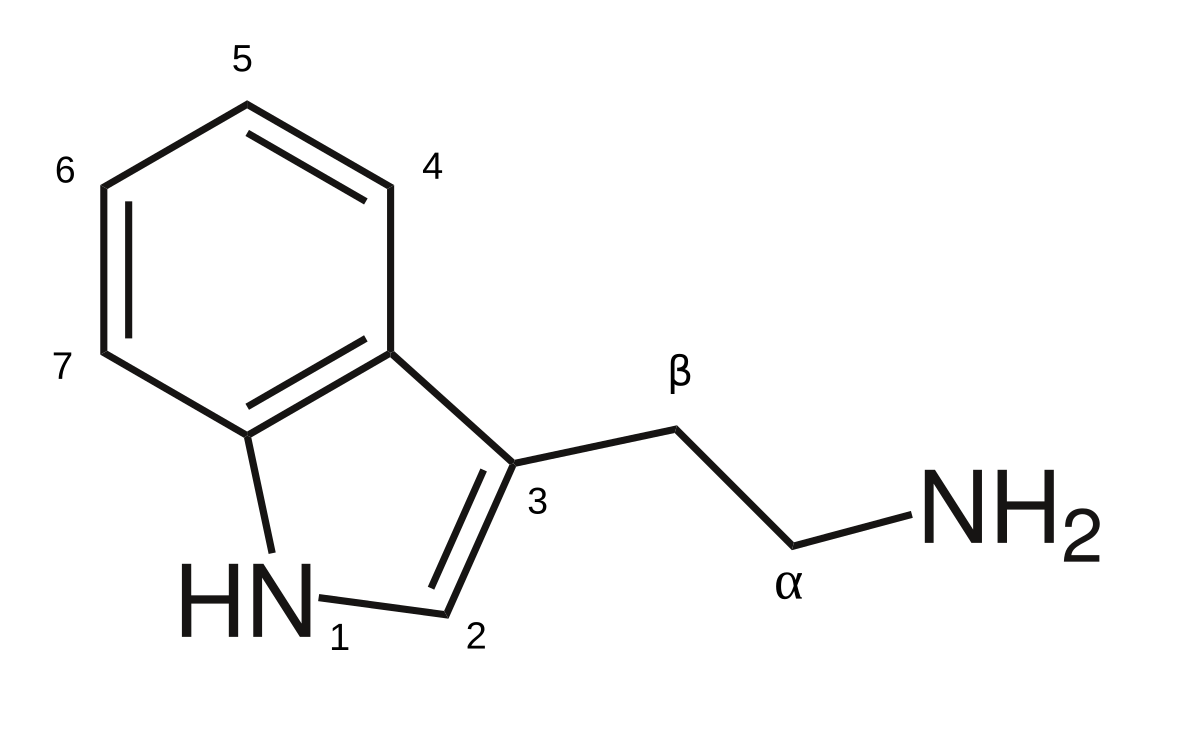
The title says it all- Antibiotics? Antibiotics are hallucinogens? I've taken antibiotics and I haven't tripped!
It is well established but little discussed that many antibiotics are capable of, in rare instances, causing a set of side effects known "Antibiotic Associated Encephalopathy" which are clinically defined as a form of neurotoxicity. A subset of Antibiotic Associated Encephalopathies are described as "Type II" or "Psychotic", according to a classification scheme offered in Bhattacharyya et al. 20161. This cluster of presents from only a few specific classes of antibiotics, which will be detailed here.
In the footnotes of my post on Mefloquine I lay out that Hallucinogen (capital H) refers to the traditional trifecta of Psychedelics-Dissociatives-Deliriants, substances that at the right dose, will consistently induce hallucinatory effects. There is the much broader term "hallucinogen" (little h) which refers to the much broader spectrum of drugs that will incidentally induce hallucinations in some cases, such as cannabinoids, alcohol, regular stimulants, or the antibiotics discussed here.
Perhaps most interesting about antibiotic induced hallucinations is that each class of drug or each specific substance seems to yield a specific image in each of their hallucinations- such as insects, worms, or helicopters. It should be noted that the visual hallucinations are almost always accompanied with a state of acute psychosis and delusional paranoia.
Several different classes of Antibiotic have been documented to cause hallucinations in some patients. Though these effects are not seen in the vast majority of patients treated with antibiotics (as they are among of the most prescribed used drugs in the world), they have a higher incidence in patients with risk factors such as being elderly, being a young child, having history of CNS damage, or having a history of kidney problems2. Overall, psychotic symptoms present in about anywhere from .9-11% (varying depending on substance) of antibiotics patients. Thankfully, in almost all cases psychotic side effects subside with cessation of the medication.
I would like to reiterate- these PSYCHOTIC effects present as a subset of clinically defined neurotoxicity- that is damage to the nervous system. This is not anything anyone should intentionally induce under ANY circumstances. The experience is a distressing delirious psychosis of which hallucinations are only one component. Furthermore, due to the low incidence of hallucinatory side effects (coupled with the fact that most incidences occurred in those with risk factors) it is likely that you cannot predict the effects well enough to reliably induce hallucinations, though you would likely rack up other negative side effects in doing so. Lastly and most importantly, taking antibiotics when not prescribed is EXTREMELY UNADVISED as any medical professional can tell you and can cause PERMANENT IMMUNE SYSTEM DAMAGE.
The different types of antibiotics that will be detailed here are Fluorouinolines, Carbapenems, Cephalosporins, Macrolides, Sulfonamides, and Penecillins.
Fluoroquinolones
 |
| A variety of Fluoroquinolone antibiotics (I was too lazy to draw my own molecules this time) |
Hypotheses for the method of action of Fluoroquinolones antibiotic induced neurotoxicity include GABA receptor inhibition and NMDA receptor agonism, though it is still not fully understood. It is also suspected that it weakens the blood-brain barrier in a way that may increase sensitivity to other medicines in a way that causes neurotoxicity1.
Specific Fluoroquinolones cited for psychotic side effects are Ciprofloxacin, Ofloxacin, Levofloxacin, Sparfloxacin, Grepafloxacin, Trovifloxacin, and Moxifloxacin2.
Let's look at some specific case studies.
Ciprofloxacin
 |
| Ciprofloxacin |
One case report cites an otherwise healthy 27 year old woman who presented with an eye infection. She was prescribed Ciprofloxacin eye drops3. She was prescribed 1 drop in each eye once an hour. 30 minutes after her third dose, she reported "well defined visual hallucinations" and "poorly defined auditory hallucinations", reminiscent of the creepy indistinct mumbling of Mefloquine. The exact nature of the visual hallucinations is not described. These symptoms subsided 24 hours after she stopped treatment.
This case report mentioned young women as being vulnerable to these psychotic side effects. One interesting component is that she could clearly remember all of the hallucinations, which is unlike the amnesiac delirium induced by drugs like anticholinergics or even other quinolines like Mefloquine.
Ofloxacin (Levofloxacin)
 |
| The (-) Isomer of Ofloxacin, Levofloxacin |
Ofloxacin is also indicated for a wide variety of bacterial infections. It is a racemic mixture of it's two stereoisomers, Levofloxacin (-) and Dextrofloxacin (+). Oftentimes, only the (-) isomer is used as a medication. As there are multiple reports of Levofloxacin inducing hallucinogens and none of Dextrofloxacin doing so, it can be assumed that the Levofloxacin isomer is wholly responsible for the hallucinations in racemic Ofloxacin.
It is one of the quinoline antibiotics most commonly cited in inducing psychotic hallucinatory side effects, though the vast majority of these cases only present in young children around the age of 4 or 5.There exist several case reports about Ofloxacin-induced psychosis.
Another case study of a 5 year old girl being treated with 50 mg twice daily of Ofloxacin presented:
"the child woke up screaming, telling that she could see large ants crawling all over the bed and in the room. She also alleged that she could see a scooter and helicopter flying in the room with bright headlights"5
This also subsided with time, and this patient could also clearly remember the course of her hallucinations.
One last case with a 6 year old boy being treated with 400 mg a day for 3 days saw:
"insects crawling all over the walls. His mother woke up and turned on the lights to find that there were none. He also complained of hearing voices which were not audible to others of a group of people who weren’t in the room. He also felt insects crawling all over his body. He also reported seeing a ghost sitting on his 10 months old sister"6
The (-) isomer, Levofloxacin, when used alone seems to only affect elderly patients. A 52 year old woman reported "seeing people who are not there"7, A 79 year old woman "became confused, irritable, and disoriented to time, place, and person. She had optic and acoustic hallucinations"8, and 50 year old man "experienced visual hallucinations of people in his hospital room. Gradually his confusion worsened and he became more violent in nature."9. A younger patient, 19 years old, reported exclusively tactile hallucinations "a feeling of insects crawling over his face, chest, and upper limbs with itching."10 All of these patients saw symptoms subside with cessation.
Something interesting about the Ofloxacin hallucinations is the common themes seen throughout- specifically patients describing seeing or feeling insects crawling on them and oddly enough, seeing helicopters. Hallucinated insects or spiders are commonly reported with anticholinergic deliriants like DPH or Scopolamine. This is interesting as Quinolines are not known to have anticholinergic activity. This may suggest that multiple receptor pathways are able to induce a similar delirious state.
Carbapenems
Carbapenems are antibiotics typically reserved for treatment resistant bacterial infections. They are classified as β-lactams, a family of antibiotics defined by their binding to the penicilin sites on bacteria, destroying their cell walls.
The neurotoxic effects of Carbapenems are most commonly unerstood as an epilepsy-like encephalopathy. Psychotic symptoms and hallucinations only present in a fraction of the few cases in which neurotoxic effects are seen. This is likely also a result of GABA inhibition/NMDA agonism1.
The specific substance cited for hallucinations in this family is Ertapenem.
Ertapenem
Ertapenem (Invanz) is prescribed for a variety of bacterial infections. While multiple case studies vaguely cite hallucinations as a component of Ertapenem induced psychosis, such as one with 1g daily dosage11. One other case study however features more vivid descriptions of the experience. In this case, the patient was a 58 year old man, who described:
"On multiple occasions, the presence of his close friends was perceived as real and he engaged in sensible conversation, only to notice their actual absence after a brief period of time. Others episodes included seeing text messages on his switched-off cell phone, and pouring tea into an absent cup, again, noted by the patient shortly afterwards. These were also well noted by family members and were initially thought to be generalised “confusions”, to the degree that the patient was constantly unsure if he was hallucinating or not. He was not delusional, remained conscious throughout and recalled these episodes in vivid detail as real events, which caused significant distress to him and the family."12
The symptoms typically presented about 4 hours after a dose of Ertapenem, and per usual symptoms went away after discontinuation. It was observed that they returned when doctors attempted to put him on a lower dose of the drug.
Cephalosporins
Cephalosporins are another class of β-lactams antibiotics that have a similar mechanism of action to penicillin. They are derived from the fungus genus Acremonium, formerly called Cephalosporium, from which they get their name. Oddly enough, the fungus and resulting compounds were first discovered in a sewage outfall in a harbor- not the type of place you'd expect a livesaving antibiotic to come from. They are indicated to treat a few specific species of bacteria vulnerable to this type of compound. Cephalosporin neurotoxicity typically presents as seizures, though in rare cases, psychotic hallucinatory delirium can occur13. They present most frequently in patients with decreased kidney function.
Macrolides
Macrolides are molecules based on a very large ring, with 14-16 members, to which 2 sugars are attached. They are used to treat respiratory and soft tissue infections (one of which, Azithromycin, has also been dubiously reported by certain demographics to be a component of a "miracle cure" cocktail of drugs for COVID 19). The mechanism of action for Macrolide neurotoxicity is suspected to be downregulation of CYP3A4, typically as a result of the chemical not being properly filtered by the kidneys. This may lead to a buildup of the unmetabolized drug, which may in turn cause psychotic effects through some unknown mechanism15. This is still not wholly understood and is for now, mostly conjecture.
Psychotic effects are primarily seen in Clarithromycin, and in extremely rare cases, Erythromycin15.
Clarithromycin
Clarithromycin is another essential medicine used for a variety of serious bacterial infections. The delirious hallucinations that present as in Clarithromycin psychosis seem to share a common element of involving swarms of worms. In one case study, a 71 one year old woman taking 500 mg of Clarithromycin reported, on the 16th day of her dosage regimen, "seeing worms over her body and in her surrounding
environment".15 Another 49 year old male patient also reported hallucinating worms13. As in previous cases, this subsided with the cessation of the medication
Sulfonamides
The Sulfonamide is a very basic functional group that is featured in the structure of a wide variety of drugs with a wide variety of uses. They are often broadly referred to as Sulfa drugs. The first ones developed were antibacterial antibiotics however. Sulfonamides are not prescribed as frequently as other antibiotics due to a relatively high incidence of allergies or side effects. Psychotic side effects are very are and have only been seen in one medication- a combination of Trimethoprim and Sulfamethoxazole (TMP-SMZ). The mechanism for how this medication induces psychosis is still unknown. One hypothesis suggests that it's connected to how the drugs inhibit metabolism of folic acid16.
Trimethoprim-Sulfamethoxazole
Trimethoprim-Sulfamethoxazole (TMP-SMZ) is a combination antibiotic prescribed for a variety of bacterial infections. Both drugs are very rarely prescribed on their own, as they are both known to work best in the presence of the other. In this combination, Sulfaethoxazole is the sulfonamide likely responsible for the psychiatric side effects, though it isn't prescribed on its own frequently enough to determine whether it bears the sole responsibility for that. TMP-SMZ is notorious for its psychotic effects, both in their acuteness and frequency. One study found that 11.9% of HIV patients being treated with TMP-SMZ for pneumonia had psychotic side effects17.
A 74 year old woman being treated with TMP-SMZ (160 mg/80 mg) twice daily began to wander from her room, and within 3 days she reported:
" seeing artificial snow outside her window, felt that the hospital was falling apart, and described seeing plaster falling off the walls."18
In another stranger case, an alarmingly young 19 year old woman with a history of spina bifida was found:
"staring at the wall of her hospital room and shouting inappropriate words while seeming to follow imaginary objects on the wall with her eyes."19
Perhaps the most alarming case report however, was an 18 year old male who had attempted suicide with a gunshot to the face. This was after beginning a regiment of TMP-SMZ for an infection.
"On the day of admission, the patient “saw” his deceased paternal uncle, himself a victim of suicide. The vision audibly reassured him, “It is okay to shoot yourself.” Shortly afterward"16
This last case particularly highlights the massive dangers in medications with psychotic side effects. Psychosis can push people into paranoid delusional states where they harm themselves or others. This is further exacerbated if it occurs alongside already existing mental illnesses.
As with all other antibiotics, symptoms subdsided with the cessation of the medication.
Penicillins
Penicillin is well known as one of the first and most widely used antibiotics to be developed, derived from the Penecillium food molds. Because of its widespread use, many bacteria have developed resistance to it, and it has a relatively high incidence of allergies, thus a whole plethora of other antibiotics, including Penicillin analogues, have been developed since.
Psychotic side effects in Penecillins have a range of causes. In Penicillin G Procaine, the psychosis inducing agent is most likely the procaine, due to its pharmacological similarity to Cocaine, and may be akin to a sort of stimulant psychosis (minus the contribution of sleep deprivation)1. As Penicillins are classified as β-lactams, the other psychiatric effects they have can be attributed to the same method of action as the Carbapenems and Cephalosporins. We will look specifically at Penicillin G Procaine, and Amoxycillin. Ampicillin is also cited to produce hallucinations though there were few case studies on it.
Penicillin G Procaine
Penicillin G Procaine is another combination antibiotic. Penicillin G Procaine is an injectable drug combined with procaine (also known as novocaine), a local anesthetic, as penicillin can cause pain at the site of injection.
As mentioned before, Penicillin on its own doesn't present with Psychotic side effects. However, the Penicillin G Procaine cocktail is well established as doing so- so established in fact that the effect is referred to as "Hoigne's Syndrome". In one case study, a 3 year old girl receiving injections reported:
" bugs on her arms and snakes in the room. She attempted to remove bugs from her body. These hallucinations continued, with several brief interludes, all night"20
This closely matches the hallucinations seen in all of the other antibiotics. It's curious that animals humans seem to viscerally fear like snakes and insects and spiders seem to present so commonly in these delirious states. In another case, a 49 year old male reported:
" that the normal sounds in the emergency room became amplified with a reverberating quality, and he had a strong feeling of impending doom. He thought a male nurse wore facial make-up and had paper-mach6-like skin on his arms. Inspection of his own arms revealed that they too had an artificial-appearing texture. Visual illusions and hallucinations stopped within twenty minutes of onset. He then thought the staff were alien beings involved in a conspiracy to use him for organ transplants and drug trials."21
This case study details the extensiveness of psychotic effects and how acutely they can affect every part of the patient's existence. It is worth remembering that psychotic antibiotic effects aren't only hallucinations, but full-blown paranoia and delusions.
Amoxicillin
Amoxicillin is another multi-use antibiotic that is very widely prescribed. It has the same mechanism of action as other penicillins. What is interesting about amoxicillin is that it induces hallucinatory side effects independent of an additive like Procaine. This suggests that Penicillin may still be in part responsible for the hallucinatory effects of that combination courtesy of its action as a β-lactam. Unfortunately, most of the case reports on Amoxicillin induced psychosis are inacessible, even through sci-hub.
A case study worksheet presenting a hypothetical situation however, summarizes them, with patients aged 30-63 demonstrating tactile, auditory, and visual hallucinations and agitation. 22
Antibiotic induced psychotic hallucinations are no picnic, they are not any sort of recreational hallucinatory experience and don't occur frequently enough to even be considered for that role. Rather, they are a form of neurotoxicity that must be considered in clinical settings. In almost all case studies, doctors attempted to treat the psychotic symptoms with antipsychotics, with little effect. The psychotic symptoms always eventually subsided with cessation of the offending antibiotic.
Sources and Further Reading:
1-Bhattacharyya S, Darby RR, Raibagkar P, Castro LNG, Berkowitz AL (2016) Antibiotic-associated encephalopathy. Neurology 86(10)
2-Grill MF, Maganti RK (2011) Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 72(3):381‐393
3-Tripathi A, Chen SI, O'Sullivan S (2002) Acute Psychosis Following the Use of Topical Ciprofloxacin. Arch Ophthalmol. 120(5):665-666
4-Bhattacharya, A, Sharan R., Praharaj SK (2017). High Dose Ofloxacin-induced Bimodal Hallucinations in a 4 Years Old Child. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology 15(4)
5-Bisht M, Kumar A (2019) Ofloxacin Induced Visual Hallucinations in Pediatric Patient. Ann Pharmacol Pharm. 2(11): 1062
6-Desousa A, Chaudhari P, Karia S, Nilesh S (2019) Ofloxacin induced hallucinations in a 6 years old child: A case report. Telangana Journal of Psychiatry 5: 64-65.
7-Raffaelli V, Cantoni G, Nucera1 G, Marino P (2018) Levofloxacin-induced visual hallucinations: A case report and review of the literature. Journal of Health and Social Sciences 3(1):85-89
8-Kogan Y, Elias N, Paz A, Odeh M (2018) Acute Delirium Associated With Levofloxacin. Journal of clinical medicine research 10(9):725–727
9-Moorthy N, Raghavendra N, Venkatarathnamma PN (2008) Levofloxacin-induced acute psychosis. Indian journal of psychiatry 50(1):57–58
10-Maharani B, Jafrin AL, Bai, KV, Sivagnanam G. (2019) Levofloxacin-induced tactile hallucination and acute anxiety reaction. Indian journal of pharmacology 51(2):123–125
11-Sutton SS, Jumper M, Cook S, Edun B, Wyatt MD. (2017) Ertapenem-Induced Encephalopathy in a Patient With Normal Renal Function. J Investig Med High Impact Case Re. 5(1)
12-Kong V, Beckert L, Awunor-Renner C (2009). A case of beta lactam-induced visual hallucination. The New Zealand medical journal 122: 76-77.
13-Mattappalil A, Mergenhagen KA. (2014) Neurotoxicity with antimicrobials in the elderly: a review. Clin Ther. 36(11):1489‐1511
14-https://www.aao.org/eyenet/article/medication-related-visual-hallucinations-what-you-
15-Ma TK, Chow KM, Choy AS, Kwan BC, Szeto CC, Li PK (2014) Clinical manifestation of macrolide antibiotic toxicity in CKD and dialysis patients. Clinical kidney journal 7(6): 507–512
16-Parashar S, Roy N, Osuagwu FC, Khalid Z, Tinklepaugh M, Mehr S, Dillon JE (2016). Trimethoprim-Sulfamethoxazole-Induced Psychosis Culminating in Catastrophic Self-Injury: A Case Report. The primary care companion for CNS disorders 18(1)
17-https://www.uspharmacist.com/article/nonpsychotropic-medicationinduced-psychosis
18-Gregor JC, Zilli CA, Gotlib IH (1993) Acute psychosis associated with oral trimethoprim-sulfamethoxazole therapy. Can J Psychiatry 38(1):56‐58
19-Saidinejad M, Ewald MB, Shannon MW (2005) Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 115(6)
20-Robertson Jr. CR (1985) Hallucinations After Penicillin Injection. m J Dis Child.139(11):1074
21-Cummings JL, Barritt CF, Horan M (1986) Delusions induced by procaine penicillin: case report and review of the syndrome. Int J Psychiatry Med. 16(2):163‐168
22-Murphy MB, Alcera LC, Gill JK, Dunn J (2008) The Inexplicably Suicidal Patient. Current Psychiatry 7(11):73-82
The first case report presented in a 4 year old girl. She was prescribed 100 mg of Ofloxacin orally twice a day to treat gastroenteritis. She soon complained of:
"snakes in her
room and felt ants crawling up her arms which she brushed
off. She commented that her father had a helicopter in his
hand and was putting it in a bag. She reported that strangers were standing in the room and looking at her"4
These extremely distressing effects were found to be a result of the girl inadvertently being given 800 mg of Ofloxacin, an adult sized dose by any measure. Psychotic symptoms subsided after 72 hours.
Another case study of a 5 year old girl being treated with 50 mg twice daily of Ofloxacin presented:
"the child woke up screaming, telling that she could see large ants crawling all over the bed and in the room. She also alleged that she could see a scooter and helicopter flying in the room with bright headlights"5
This also subsided with time, and this patient could also clearly remember the course of her hallucinations.
One last case with a 6 year old boy being treated with 400 mg a day for 3 days saw:
"insects crawling all over the walls. His mother woke up and turned on the lights to find that there were none. He also complained of hearing voices which were not audible to others of a group of people who weren’t in the room. He also felt insects crawling all over his body. He also reported seeing a ghost sitting on his 10 months old sister"6
The (-) isomer, Levofloxacin, when used alone seems to only affect elderly patients. A 52 year old woman reported "seeing people who are not there"7, A 79 year old woman "became confused, irritable, and disoriented to time, place, and person. She had optic and acoustic hallucinations"8, and 50 year old man "experienced visual hallucinations of people in his hospital room. Gradually his confusion worsened and he became more violent in nature."9. A younger patient, 19 years old, reported exclusively tactile hallucinations "a feeling of insects crawling over his face, chest, and upper limbs with itching."10 All of these patients saw symptoms subside with cessation.
Something interesting about the Ofloxacin hallucinations is the common themes seen throughout- specifically patients describing seeing or feeling insects crawling on them and oddly enough, seeing helicopters. Hallucinated insects or spiders are commonly reported with anticholinergic deliriants like DPH or Scopolamine. This is interesting as Quinolines are not known to have anticholinergic activity. This may suggest that multiple receptor pathways are able to induce a similar delirious state.
Carbapenems
 |
| The generalized structure of a Carbapenem |
Carbapenems are antibiotics typically reserved for treatment resistant bacterial infections. They are classified as β-lactams, a family of antibiotics defined by their binding to the penicilin sites on bacteria, destroying their cell walls.
The neurotoxic effects of Carbapenems are most commonly unerstood as an epilepsy-like encephalopathy. Psychotic symptoms and hallucinations only present in a fraction of the few cases in which neurotoxic effects are seen. This is likely also a result of GABA inhibition/NMDA agonism1.
The specific substance cited for hallucinations in this family is Ertapenem.
Ertapenem
 |
| Ertapenem |
"On multiple occasions, the presence of his close friends was perceived as real and he engaged in sensible conversation, only to notice their actual absence after a brief period of time. Others episodes included seeing text messages on his switched-off cell phone, and pouring tea into an absent cup, again, noted by the patient shortly afterwards. These were also well noted by family members and were initially thought to be generalised “confusions”, to the degree that the patient was constantly unsure if he was hallucinating or not. He was not delusional, remained conscious throughout and recalled these episodes in vivid detail as real events, which caused significant distress to him and the family."12
The symptoms typically presented about 4 hours after a dose of Ertapenem, and per usual symptoms went away after discontinuation. It was observed that they returned when doctors attempted to put him on a lower dose of the drug.
Cephalosporins
 |
| Generalized structure of a Cephalosporin |
The specific Cephalosporins resposible for hallucinations are not well documented. An article about medication induced delirium cites cephalosporins as frequently being associated with antibiotic hallucinations but it doesn't clarify past that14. In fact my impetus for writing this entire article was someone in a group chat giving an anecdotal report of experiencing hallucinations from Cefdinir as a child, though I could find nothing on it in the literature.
 |
| Cefdinir |
Macrolides
 |
| Various Macrolide Antibiotics |
Psychotic effects are primarily seen in Clarithromycin, and in extremely rare cases, Erythromycin15.
Clarithromycin
 |
| Clarithromycin (Drawn slightly differently from above) |
Sulfonamides
 |
| Sulfonamide |
Trimethoprim-Sulfamethoxazole
 |
| Trimethoprim (top) and Sulfamethoxazole (bottom) |
A 74 year old woman being treated with TMP-SMZ (160 mg/80 mg) twice daily began to wander from her room, and within 3 days she reported:
" seeing artificial snow outside her window, felt that the hospital was falling apart, and described seeing plaster falling off the walls."18
In another stranger case, an alarmingly young 19 year old woman with a history of spina bifida was found:
"staring at the wall of her hospital room and shouting inappropriate words while seeming to follow imaginary objects on the wall with her eyes."19
Perhaps the most alarming case report however, was an 18 year old male who had attempted suicide with a gunshot to the face. This was after beginning a regiment of TMP-SMZ for an infection.
"On the day of admission, the patient “saw” his deceased paternal uncle, himself a victim of suicide. The vision audibly reassured him, “It is okay to shoot yourself.” Shortly afterward"16
This last case particularly highlights the massive dangers in medications with psychotic side effects. Psychosis can push people into paranoid delusional states where they harm themselves or others. This is further exacerbated if it occurs alongside already existing mental illnesses.
As with all other antibiotics, symptoms subdsided with the cessation of the medication.
Penicillins
 |
| Generalized structure of a Penicillin |
Penicillin is well known as one of the first and most widely used antibiotics to be developed, derived from the Penecillium food molds. Because of its widespread use, many bacteria have developed resistance to it, and it has a relatively high incidence of allergies, thus a whole plethora of other antibiotics, including Penicillin analogues, have been developed since.
Psychotic side effects in Penecillins have a range of causes. In Penicillin G Procaine, the psychosis inducing agent is most likely the procaine, due to its pharmacological similarity to Cocaine, and may be akin to a sort of stimulant psychosis (minus the contribution of sleep deprivation)1. As Penicillins are classified as β-lactams, the other psychiatric effects they have can be attributed to the same method of action as the Carbapenems and Cephalosporins. We will look specifically at Penicillin G Procaine, and Amoxycillin. Ampicillin is also cited to produce hallucinations though there were few case studies on it.
Penicillin G Procaine
 |
| Penicillin G Procaine |
As mentioned before, Penicillin on its own doesn't present with Psychotic side effects. However, the Penicillin G Procaine cocktail is well established as doing so- so established in fact that the effect is referred to as "Hoigne's Syndrome". In one case study, a 3 year old girl receiving injections reported:
" bugs on her arms and snakes in the room. She attempted to remove bugs from her body. These hallucinations continued, with several brief interludes, all night"20
This closely matches the hallucinations seen in all of the other antibiotics. It's curious that animals humans seem to viscerally fear like snakes and insects and spiders seem to present so commonly in these delirious states. In another case, a 49 year old male reported:
" that the normal sounds in the emergency room became amplified with a reverberating quality, and he had a strong feeling of impending doom. He thought a male nurse wore facial make-up and had paper-mach6-like skin on his arms. Inspection of his own arms revealed that they too had an artificial-appearing texture. Visual illusions and hallucinations stopped within twenty minutes of onset. He then thought the staff were alien beings involved in a conspiracy to use him for organ transplants and drug trials."21
This case study details the extensiveness of psychotic effects and how acutely they can affect every part of the patient's existence. It is worth remembering that psychotic antibiotic effects aren't only hallucinations, but full-blown paranoia and delusions.
Amoxicillin
 |
| Amoxicillin |
A case study worksheet presenting a hypothetical situation however, summarizes them, with patients aged 30-63 demonstrating tactile, auditory, and visual hallucinations and agitation. 22
Antibiotic induced psychotic hallucinations are no picnic, they are not any sort of recreational hallucinatory experience and don't occur frequently enough to even be considered for that role. Rather, they are a form of neurotoxicity that must be considered in clinical settings. In almost all case studies, doctors attempted to treat the psychotic symptoms with antipsychotics, with little effect. The psychotic symptoms always eventually subsided with cessation of the offending antibiotic.
Sources and Further Reading:
1-Bhattacharyya S, Darby RR, Raibagkar P, Castro LNG, Berkowitz AL (2016) Antibiotic-associated encephalopathy. Neurology 86(10)
2-Grill MF, Maganti RK (2011) Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 72(3):381‐393
3-Tripathi A, Chen SI, O'Sullivan S (2002) Acute Psychosis Following the Use of Topical Ciprofloxacin. Arch Ophthalmol. 120(5):665-666
4-Bhattacharya, A, Sharan R., Praharaj SK (2017). High Dose Ofloxacin-induced Bimodal Hallucinations in a 4 Years Old Child. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology 15(4)
5-Bisht M, Kumar A (2019) Ofloxacin Induced Visual Hallucinations in Pediatric Patient. Ann Pharmacol Pharm. 2(11): 1062
6-Desousa A, Chaudhari P, Karia S, Nilesh S (2019) Ofloxacin induced hallucinations in a 6 years old child: A case report. Telangana Journal of Psychiatry 5: 64-65.
7-Raffaelli V, Cantoni G, Nucera1 G, Marino P (2018) Levofloxacin-induced visual hallucinations: A case report and review of the literature. Journal of Health and Social Sciences 3(1):85-89
8-Kogan Y, Elias N, Paz A, Odeh M (2018) Acute Delirium Associated With Levofloxacin. Journal of clinical medicine research 10(9):725–727
9-Moorthy N, Raghavendra N, Venkatarathnamma PN (2008) Levofloxacin-induced acute psychosis. Indian journal of psychiatry 50(1):57–58
10-Maharani B, Jafrin AL, Bai, KV, Sivagnanam G. (2019) Levofloxacin-induced tactile hallucination and acute anxiety reaction. Indian journal of pharmacology 51(2):123–125
11-Sutton SS, Jumper M, Cook S, Edun B, Wyatt MD. (2017) Ertapenem-Induced Encephalopathy in a Patient With Normal Renal Function. J Investig Med High Impact Case Re. 5(1)
12-Kong V, Beckert L, Awunor-Renner C (2009). A case of beta lactam-induced visual hallucination. The New Zealand medical journal 122: 76-77.
13-Mattappalil A, Mergenhagen KA. (2014) Neurotoxicity with antimicrobials in the elderly: a review. Clin Ther. 36(11):1489‐1511
14-https://www.aao.org/eyenet/article/medication-related-visual-hallucinations-what-you-
15-Ma TK, Chow KM, Choy AS, Kwan BC, Szeto CC, Li PK (2014) Clinical manifestation of macrolide antibiotic toxicity in CKD and dialysis patients. Clinical kidney journal 7(6): 507–512
16-Parashar S, Roy N, Osuagwu FC, Khalid Z, Tinklepaugh M, Mehr S, Dillon JE (2016). Trimethoprim-Sulfamethoxazole-Induced Psychosis Culminating in Catastrophic Self-Injury: A Case Report. The primary care companion for CNS disorders 18(1)
17-https://www.uspharmacist.com/article/nonpsychotropic-medicationinduced-psychosis
18-Gregor JC, Zilli CA, Gotlib IH (1993) Acute psychosis associated with oral trimethoprim-sulfamethoxazole therapy. Can J Psychiatry 38(1):56‐58
19-Saidinejad M, Ewald MB, Shannon MW (2005) Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 115(6)
20-Robertson Jr. CR (1985) Hallucinations After Penicillin Injection. m J Dis Child.139(11):1074
21-Cummings JL, Barritt CF, Horan M (1986) Delusions induced by procaine penicillin: case report and review of the syndrome. Int J Psychiatry Med. 16(2):163‐168
22-Murphy MB, Alcera LC, Gill JK, Dunn J (2008) The Inexplicably Suicidal Patient. Current Psychiatry 7(11):73-82