For today's installment we will be looking at a unique series of tryptamines- these are the tricylic tryptamines, also known as "conformationally restrained" tryptamines, as their are tightly bonded to themselves in several places and this prevents the molecule from twisting or turning in any ways. I ramble about the implications these compounds have in understanding the structure-activity relationship between psychedelic substances and our 5-HT2A receptors. If you're here just to learn about the substances themselves you can scroll past that.
 |
| The generalized structure of a tricylic tryptamine |
There are some interesting things to note about this molecule- the first is that it appears to be half of the lysergamide molecule, the family of molecules to which drugs like LSD, ALD-52 and LSA belong.
 |
| The tricyclic tryptamine highlighted within the generalized structure for a lysergamide |
The second observation is that the tricyclic tryptamine structure (and by extension the lysergamide structure) contains the general forms for the two major families of psychedelic, the tryptamine and the phenethylamine (though the group as a whole is named for the more prominent tryptamine base tot the structure)
 |
| The structures for a tryptamine (left) and a phenethylamine (right) hiding out in the structure of a Tricyclic tryptamine |
This raises an important question in drug discovery- what molecular forms are responsible for triggering the psychedelic experience? So far there are 3 classes of molecule known to trigger a psychedelic experience via agonism of the 5-HT2A receptor primarily: The tryptamines, the phenethylamines, and the lysergamides (which, as shown here, technically contain the other two groups). (side note, a 4th class, the piperazines, are sometimes included, as they can sometimes have some minor agonism on 5-HT2A, though these drugs are more commonly recognized primarily as stimulants). So what does it take for a molecule to be recognized by the 5-HT2A receptor in a way that triggers a psychedelic experience?
The legendary David Nichols published a seminal analysis of the structure-activity relationship of psychedelics in 20121. The only common element shared by the three major groups of psychedelics is an ethylamine group attached to some sort of aromatic ring structure, with at least 1 phenyl ring involved somewhere in the structure. He observes that several minor downstream modifications beyond that can render the compound inactive, like a Fluorine on the 6 position of a tryptamine, or the reduction of the double bond between the 9 and 10 positions on a lysergamide. In tryptamines, modifying the base rings by replacing them with thiophene, benzofuran, or pyrazole groups yielded compounds with notable receptor activity, suggesting they aren't entirely inactive though whether they are psychedelic is unknown. Phenethylamines meanwhile retain activity even when strapped with all sorts of massive modifications like multiple benzofuran rings attached to the phenyl ring, or entire subsituted phenyl structures attached to the ethylamine nitrogen. This paints an extremely complicated picture where the 5-HT2A receptor is at once very fussy yet broadly selective in what it accepts as agonists. So long as certain aromatic rings provide a stable base for an ethylamino group without other side groups interfering with receptor function, it seems it will accept a molecule and trigger a psychedelic experience. Tricyclic tryptamines check all these boxes, and thus will readily function as psychedelics. But I digress, what are these drugs actually like? We will be looking at 2 substances within this class that have a recorded history of human usage. RU-28306 and NDTDI.
RU-28306
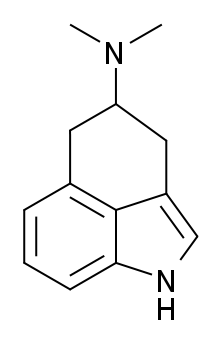 |
| RU-28306 |
RU-28306 is first mentioned in a receptor assay from 1981 by Euvrard et al. It was first studied as a dopamine agonist associated with the treatment of Parkinsons. This study mostly just determined that RU-28306 probably isn't the best for treating Parkinsons and that it may also briefly inhibit lactation. It was determined that at the very least, medical doses of RU-28306 were higher than medical doses of the known substance Bromocriptine, which doses at ~1 mg2.
A later study by EW Taylor et al. however, looked into its effects on 5-HT receptors. This wasn't from the perspective of trying to find new receptor agonists, but rather in this study RU-28306 was simply a tool being used to better characterize the action of 5-HT receptors. This was part of a larger effort to learn more about antipsychotics that acted on the 5-HT receptors like Buspiridone. In this study, RU-28306 demonstrated a higher affinity for the 5-HT2 receptor than DMT, and a lower affinity for the 5-HT1A receptor. The authors conclude that this suggests RU-28306 has an equal or higher potency than DMT3. I conclude that this perhaps shows promise as a psychedelic, though that of course can't be known until it's tested in humans.
Fast forward almost 30 years and RU-28306 appears in a classic "small and handy" bluelight thread. Forum members speculate on the substance for 3 years until its entry into the market is announced.
Finally, a user announces obtaining it only to describe a purplish, gaseous/powdery substance suspended in the air of the bag, impossible to photograph. Mysterious. This user speculated doses of ~1 mg but never returned to the thread.
Another user found a trip report, translated from German, for 800 μg insufflated, which seemed to also suggest a very high potency for this substance. This reported pointed to it being a weaker trip, with sedative and motor-inhibiting effects and sexual enhancement. It seemed to induce uncontrollable sleep in the user, who expressed concern that it could be used as a date-rape drug and gave a negative review to the vendor.
This is all the information that could be find. Many people reported obtaining this substance, but almost none of them gave any reports. As I said about 4-HO-DBT, if you obtain a substance with 0 experience reports and you try it, you are OBLIGATED to write a report, for the sake of the community.
It was noted by many users that this substance was exceptionally expensive despite its high potency, and a difficult synth was cited as the reason for this. It is unlikely it will ever be seen again. Users also noted how light and fluffy and chalky the powder was and that it had a tendency to fluff up and escape the bag when handled.
NDTDI
 |
| NDTDI |
NDTDI looks like some twisted chimeric attempt to graft LSD onto the tricyclic tryptamine structure, or rather it looks like LSD with the 9-position carbon plucked from the structure. Nichols in his paper mentioned that the loss of the 9-10 double bound rendered LSD inactive and the carbon that forms that bond has been completely removed in NDTDI, raising questions about its activity. However, the entire structure branching from the nitrogen, which is normally constrained by a ring in LSD, is free to wiggle around in NDTDI, which may present unique effects. How did it turn out? We once again turn to a bluelight "small and handy" thread.
The results that trickled in showed little promise. The chemical was approached with caution, and people titrated up from very low doses. Eventually doses reached the range of 10-20 mg, with no effects beyond placebo noted. This substance was also exorbitantly expensive, and ultimately proved very disappointing. This raises questions as to why it was manufactured in the first place? There was promise shown with NDEPA's which similarly mimic LSD's structure by breaking the rings and giving long unconstrained chains. Active NDEPA's (which will be featured in a later installment!) lack similar elements as NDTDI, including that 9-10 double bond. Perhaps that inspired NDTDI to be produced, and perhaps it isn't specific features missing relative to LSD that have killed NDTDI, but a combination of factors in the molecule- if the cyclohexane ring and the 9-10 bond are broken, the chemical is still active, yet if the 9-10 bond is broken with the rest of the molecule intact, it's inactive. I think it would at least be interesting to see a receptor assay with this substance to confirm what exactly is going on with it.
 |
| an NDEPA, pretty much the lysergamide structure with most of its rings cut open |
Despite its seeming inactivity, NDTDI has been made illegal in Slovenia, where it's structural similarity to LSD is noted, as is its uhh... potential for... "serious harm to personal health, danger to life and danger to public safety in general" .... Another resounding victory for the war on drugs....6
Wait, that's cheap, I'm including a substance that's not actually active? That sucks! That doesn't warrant inclusion! This isn't interesting and more importantly, you the reader cannot trip on it :(
Correct. And RU-28306 isn't the most promising either. Nonetheless, I think tricyclic tryptamines still function as a discrete class of molecule, and it was worthwhile to analyze the 2 that have been tested in humans to try and draw conclusions on the patterns of effects in these substances (which by the way, showed initial promise through receptor studies!). The conclusion is that they retain a very high potency, but with a seemingly low ceiling for interesting effects, with RU-28306 being an odd sort of sedative despite being a 5-HT2A agonist. It's worthwhile to consider these pitfalls in the search for new substances, and that is why I am writing on them. Plus I got to ramble for awhile about structure activity relations.
Correct. And RU-28306 isn't the most promising either. Nonetheless, I think tricyclic tryptamines still function as a discrete class of molecule, and it was worthwhile to analyze the 2 that have been tested in humans to try and draw conclusions on the patterns of effects in these substances (which by the way, showed initial promise through receptor studies!). The conclusion is that they retain a very high potency, but with a seemingly low ceiling for interesting effects, with RU-28306 being an odd sort of sedative despite being a 5-HT2A agonist. It's worthwhile to consider these pitfalls in the search for new substances, and that is why I am writing on them. Plus I got to ramble for awhile about structure activity relations.
Sources and further reading:
1-Nichols DE (2012) Structure–activity relationships of serotonin 5‐HT2A agonists. WIREs Membr Transp Signal 1: 559-579.
2-Euvrard C, Ferland L, Fortin M, Oberlander C, Labrie F, Boissier JR (1981) Dopaminergic activity of some simplified ergoline derivatives. Drug Dev. Res.1: 151-161.
3-Taylor EW, Nikam S, Weck B, Martin A, Nelson D. (1987) Relative selectivity of some conformationally constrained tryptamine analogs at 5-HT1, 5-HT1A and 5-HT2 recognition sites. Life Sci. 41(16):1961‐1969.

No comments:
Post a Comment