[AS OF 3/20/2023, THIS ARTICLE IS MORE OR LESS RENDERED OBSOLETE WITH THE PUBLICATION OF MICHAEL DYBEK PH.D'S DOCTORAL DISSERTATION, "STRUCTURE ACTIVITY RELATIONSHIP (SAR) STUDIES OF 1,2-DIARYLETHYLAMINES AS N-METHYL-D-ASPARTATE RECEPTOR ANTAGONISTS"; THIS PUBLICATION DEFINITIVELY MAPS OUT THE EFFECT THAT VARIOUS SUBSTITUTIONS HAS ON DIARYLETHYLAMINES IN TERMS OF NMDA RECEPTOR AFFINITY. IT IS COMPREHESIVE AND I DON'T FEEL LIKE REWRITING THIS ARTICLE. I SUMMARIZED THE FINDINGS OF THAT DISSERTATION HERE. IT IS INCREDIBLY EXCITING TO HAVE THESE HYPOTHESIS AND SEE THEM TESTED THROUGH THE WORK THAT DR. DYBEK HAS DONE OVER THE PAST FEW YEARS!]
[EDITED 1/2/21 to include information the diarylethylamine stimulant A-D2PV]
The Diarylethylamines are a class of chemical best known for producing a series of fascinating dissociative hallucinogenic compounds. 3 of these compounds-Ephenidine, Diphenidine, and Methoxphenidine, were widely produced and were widely available for a time. (Some others, such as Fluorolintane and Isopropylphenidine were known to exist too, though they saw limited availability and little data exists for them). There exist some other compounds with the basic Diarylethylamine backbone that appear to have activity at other receptors, such as µ-opioid agonism or stimulant activity.
The basic structure of the Diarylethylamine is an
aromatic ring, with an ethylamine side chain and another aromatic ring at the
α-position of the side chain. Almost all known examples of diarylethylamines
see two phenyl rings as the aromatic rings, though psychoactive activity can
still possibly be retained with other aromatic rings. With a phenyl ring, a
diarlethylamine is in fact a type of phenethylamine!
Diarylethylamines respond to modifications in a fairly
predictable pattern, similar to the arylcyclohexylamines (which I have written about at length before), though a lot is yet
to be understood and as such, all sorts of SAR surprises pop up.
 |
| Generalized structure of a diarylethylamine, with phenyl rings as the aromatic rings. Some amine is placed at the blue line |
The
first diarylethylamines to be tested for their pharmacological properties were
a series of diphenyl compounds with either a primary amine, various alkane
chains as secondary amines (including Ephenidine and Isopropylphenidine), and
4-MeO-Substituted versions of the primary amine along with Ephenidine. They
were evaluated for their stimulant properties, as at the time (1943),
dissociative NMDA antagonists had not been formally identified or described yet
[11]. Most compounds in this development series would likely be active as
dissociatives nonetheless.
Diarylethylamine
development was dominated for the rest of the century by lefetamine, which saw
abuse in Japan and Europe briefly. The action of lefetamine was never fully
defined, though it is suggested to be a partial opioid agonist, and in some
manner a stimulant [4]. It is possible it may also be an NMDA antagonist (more
on that later). As the defining structure for the series, other
diarylethylamines were for a while referenced in literature as analogues of
lefetamine. Dissociative compounds were revisited in the 80’s and 90’s for the
same reason many dissociative NMDA antagonists were investigated in medicine-
as anticonvulsants, antparkinsonians, anti-ischeimics, or as neuroprotectives
[2]. This is likely the same literature that would guide the development of the
first diarylethylamines to hit the streets. Diarylethylamines as recreational
drugs were first identified in 2008 in a seizure by German police, where
Ephenidine and Isopropylphenidine were discovered. Interest in these compounds
would explode in 2013 however, following a UK ban on arylcyclohexylamines that
was put in place to stamp out MXE [12]. Ephenidine, Diphenidine, and
Methoxphenidine would inundate the online markets, see widespread use and
abuse, and eventually, as of 2020, fall out of favor and become scarce.
Fluorolintane, and Isopropylphenidine were available at some point, though
they did not see widespread sale or use and very little information exists
about them in internet drug communities [18]. A Methylethylphenidine, billed as
N-methylephenidine or “Ephenidine 2” also supposedly existed at some point,
though a single report states that it didn’t have NMDA antagonist effects.
[20].
 |
| A selection of existing Diarylethylamines that have shown NMDA antagonist activity |
So what exactly are the Diarylethylamines like? Why did they wane in popularity? There were several distinct properties they had that set them apart from many of the diarylethylamines- First was bioavailability, particularly that they were not active intranasally, a preferred method for many dissociative users. They had to be taken orally or rectally, or possibly by IV. Second is their potency- most of these compounds saw active doses upwards of 100 mg- not the most cost effective for the producer or the consumer. (This is odd because many have a higher in vitro affinity for the NMDAr receptor than PCP! Are pharmacokinetics to blame? It is not fully understood yet) [14]. Lastly, they had an effect similar to psychedelics where they would instill a cross tolerance against other NMDA receptors for about a week, which was a turnoff for frequent users. However, they also saw acclaim for their euphoric, hallucinogenic, and stimulant properties. Many remarked on the powerful compulsive redosing instilled by vaporizing Diphenidine. Ephenidine received the best reviews of them all, praised for its sociability, euphoria, visuals and general sensation. The dissocation of the diarylethylamines was characterized as “smooth” and “sinking”, without as much of a stimulant edge as the Arycyclohexylamines for most users. This author personally found value and interesting properties in all 3 of those compounds. Nonetheless, all of this information comes from the sample size of the 3 compounds that were actually produced for market though. Perhaps other unique properties are hiding out there with other compounds.
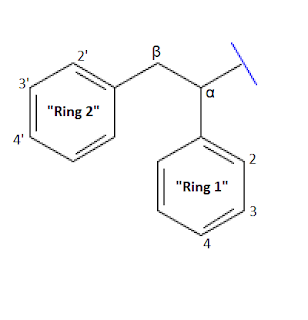 |
| Generalized Diarylethylamine structure with 2 phenyl rings and positions labeled |
So where to go for developing these? Well, they have remarkably similar properties to the arylcyclohexylamines in terms of how you can modify them yet retain activity. The nitrogen appears to accept all variety of amine substitutions, primary, secondary and tertiary, potentially the same variety of amines that could possibly be used for arylcyclohexylamines [1]. It should be noted that there appear to be pitfalls in this regard and there may be a wide range of unexpected patterns. For example, a non-cyclic tertiary amine seems to reduce NMDA antagonist activity and present partial opioid activity, as seen in Lefetamine [5]. A single report of a tertiary Methylethylamine suggested the compound was a stimulant and had no NMDA antagonist activity [20].
Then there’s the aromatic rings- most commonly
presented as phenyl rings. It is possible however that other rings could
conserve activity in that position [6]. Speaking strictly of phenyl rings
however, similar to the arylcyclohexylamines, they can potentially see
substitutions- at their 2, 3, and 4 positions. It would appear that activity is
best conserved via substitutions at the 2 position of ring 1 and the 3’
position of ring 2 (At least in the unique compound fluorolintane, which has a
pyrollidine as the amine) [13]. Substitutions at other positions on these rings
may be inactive, at least with a pyrollidine. It is also alleged that 2’
position substitutions on ring 2 are active, though the evidence for this is
conflicting and dubious [12][18]. These are not hard, fast rules though and
there are already exceptions that may render these not even rules in the first
place- or perhaps different sets of rules apply depending on what the amine is-
for example a 3-Methoxy substituted version of Diphenidine retained appreciable
activity and potency [14]. Oddly enough, a 2-Cl Diphenidine showed to be even
more potent than its parent compound, and one of the higher in vitro affinity compounds
out there [14]. So it really is hard to predict, and as mentioned before, with
diarylethylamines, in vitro affinity does not translate directly to in vivo
potency.
As for which substitutions work? Hypothetically any
that have been seen to retain or potentially retain activity in
arylcyclohexylamines, though only halogens and methoxy groups have been
attempted so far. It would be exciting to see others! Perhaps simple alkane
chains and hydroxy groups would also show promise.
So a quick summary if you want to design a new diarylethylamine:
1. The amine has proven to be active with an N-ethyl, N-isopropyl, piperidine, and pyrollidine. Thus it can be presumed that longer secondary amine chains and other tertiary amine rings will work. Noncyclic tertiary amines appear to have less receptor selectivity. A primary amine is also likely accepted [1].
2. The aromatic rings will definitely provide the most predictable results when both are phenyl rings. Other aromatic rings may work too, though this requires further study. Information on modifying the aromatic rings can be found in Mealing et al. 2001 [6] and Massa et al. 1989 [5].
3. Substitutions seem to work best on the 2 and 3’ positions of phenyl rings, (or on the 3 position if you have a piperidine instead of a pyrollidine for the amine- tons more exceptions like this may exist!). Substitutions such as halogens and methoxy groups have proven active- it is possible that other substitutions like alkane chains, hydroxy groups, amino groups, and others may conserve activity too! It is also alleged that substitutions on the 2’ position may be active, though the evidence for this is conflicting. [13][18]
4. It appears possible to add addition moieties on the α and β positions of the ethylamine side chain. In vitro, it has so far been demonstrated that an α-methyl and a β-amino group have an appreciable affinity for the NMDA receptor [1]. OH- substitutions were demonstrated to be inactive so it’s hard to say what else may or may not work in those positions. It seems a β-ketone produces a pure stimulant, as it builds a structure resembling a cathinone. This is seen in the compound A-D2PV.
5. As far as stereochemistry is concerned, it would appear that the (S)-enantiomer of any diarylethylamine will have a higher affinity for the NMDA receptor than the (R)-enantiomer. [1] [9]
This is a lot of words- perhaps seeing it mapped out
again will help visualize the door to all of the possibilities!
But first of course, a word of warning: As with any
new class of drugs, extreme caution is warranted when developing and testing
Diarylethylamines. Known risks specific to this class of chemical include
overdose [22], Carcinogenic properties [23], and nonselective receptor activity
such as opioid agonism, which could lead to other forms of overdose [4].
NMDA antagonists in general will also present a set of
risks. For ease, I am simply going to copy the warning given in my post on
arylcyclohexylamines. The cited sources in the following block of text can be
found on the page for that article.
“There are also a number of ways chronic toxicity is
reported to present with NMDA antagonists, mainly neurotoxicity and
cytotoxicity. In terms of neurotoxicity, there are the infamous Olney’s
lesions, a form of brain damage, that has been observed in other animals, though
they have still not officially been observed in humans yet [43]. However, a
recent study reportedly observed some form of damage in the brain of extremely
frequent users of ketamine [44]. The other main reported symptom that indicates
toxicity is urinary toxicity [45, 46], supposedly a result of damage to the
epithelial cells lining the bladder caused by direct toxicity from ketamine
metabolites. This has so far only been officially reported with ketamine,
though there are anecdotal reports of it occurring in frequent users of other
dissociatives. There is also a potential for cognitive dysfunction from extreme
repeated use of dissociatives, mostly in the form of “brain fog” and memory
loss, though there is some literature on the matter [47].
These substances also carry the risk of generating
dangerous behaviors that can be damaging to one’s life circumstances and
relationships, both through the dangerous interplay of prohibition and
substance, and in properties inherent to the chemicals themselves. One key risk
is addiction- while physical dependence to dissociatives is significantly more
rare than with other classes of substance, it is entirely possible and
psychological dependence is commonly reported. Frequent usage significantly
increases the chance of toxic effects or cognitive dysfunction presenting.
Other substances, such as PCP, are notorious for causing intense mania that can
push into psychosis, which can lead to violence, damaging relationships, and
legal trouble. All of these risks are real and it is up to the user to
determine what methods personally work best for mitigating them, including
total abstinence if necessary.
I would suggest, in a perfect world (keep in mind this
is all very handwaved, this actual process can be expensive, difficult, and
extremely time consuming)- First, doing a virtual docking simulation of the
compound. This of course is not a surefire way to determine activity, but can
perhaps give warning of possible unexpected activity or help to rule out
certain options as being less viable. The compound can be synthesized from
there, at which point it must be properly characterized via NMR and GC/MS
analysis. From there, an in-vitro receptor affinity study can be done to
confirm or deny certain targeted activities in nerve cells in comparison to
familiar reference compounds, like PCP, Ketamine, MK-801 or Morphine. The
safest step from there would be in-vivo studies in animals, also compared to a
control group of reference compounds. Behavioral tests can be done for comparison
to any references, and drug substitution tests can help indicate similarity to
the references. There is a huge variety of animal tests that can be done in
combination with each other and with various controls to really narrow down
possible mechanism of action depending on what a researcher has at their
disposal. In-vivo tests also help to determine an mg/kg dosage range and
possible acute or chronic toxicity, or even an LD-50. Only after it has been
presumed nontoxic and its likely activities have been characterized should one
even consider human testing. This must also be done in the context of extremely
precise doses, titrated upwards from a microgram range, with the subject
physically monitored by a healthcare professional. If you want to get really fancy,
this can be performed in a double blind test with a placebo.
Of course not all those processes or resources are
available to every researcher. Those are all long, difficult, expensive
processes that may require specialty equipment, facilities, and faculty. Many
researchers of psychoactive compounds have opted to skip some or most or all of
those steps, and prohibition absolutely makes obtaining any of those resources
extremely difficult. I would recommend approaching with maximum caution, but
I’m also not the boss of anyone and can’t make anyone do anything, and
understand how the spirit of curiosity can sometimes overcome a lack of
available resources. Ideally a team of researchers could easily have
infrastructure to efficiently run multiple compounds through that gauntlet of
safety determinations. But this world is less than ideal. Please just for the
love of god, be safe, be smart, be responsible.”
For a larger image: https://i.imgur.com/v7rVSoR.png
To download a pdf: https://gofile.io/d/tYPnG1
Some things that you may have noticed in this chart-
First is the exclusion of many of the amines that I used in my
arylcyclohexylamine chart- this is simply due to how little knowledge currently
exists for the diarylethylamines. I cut it down to which ones seem to have a
higher likelihood of being selective NMDA antagonists, while leaving out many
of the more experimental variations that have hardly even been tested on the
better understood arylcyclohexylamines. I also only left the option for
substitutions for the 2,3, and 3’ positions on the phenyl rings (As well as
some 2,3-cyclic substitutions), as substitutions at other positions, as far as
we understand, render the compound inactive. Speaking of aromatics, I leave the
option to exchange the default phenyl rings in the structure for aromatics
known to conserve activity in the arylcyclohexylamines. Not enough data exists
to even conjecture on then adding substitutions to those, though one study
suggests other aromatics will accept a methyl substitution at times [6]
Moieties that have demonstrated some sort of activity in vivo or in vitro are cited on the chart.
As for nomenclature? To copy the blurb included in the
chart-
There are no hard fast rules for naming
diarylethylamines, just like with any class of drug. Lefetamine, Lanicemine,
Remacemide, Fluorolintane, are all names of compounds in this class that don’t
seem to follow any convention. The advent of commercially available
dissociative diarylethylamines brought about the -phenidine naming scheme,
which for now stands as the most recognizable way to name possible dissociative
compounds. If you are using the chart, the amines are labeled with prefixes
that can be appended to the “-phenidine” suffix. Denoting the presence of
substitutions is as easy as simply appending the positional number and an
abbreviation of the moiety, as seen in the arylcyclohexylamines. For example, a
compound could be named 2-F-Morpholidophenidine, or 3’-EtO-EtHydroxyphenidine.
Of course in popular usage, exceptions crop up everywhere. 2-MeO-Diphenidine
was sold on the market as simply Methoxphenidine, abbreviated MXP (likely to
market it as being similar to the recently banned and immensely popular MXE). As
for instances where one of the standard phenyl rings is replaced with a
different aromatic name, the -phenidine suffix will not suffice. I suggest just
amending that suffix depending on the ring. If there are 2 thiophene rings for
example, -dithiophenidine would be a usable name. If there is say, a thiophene
+ a phenyl, then thiophenidine could be a valid name. I am still unsure of how
to denote which ring is which in this naming scheme however- perhaps the first
name listed could represent ring 1, with the second ring as ring 2. So
“Thiophenidine” would denote that ring 1 was a thiophene, while
“Phenithiophenidine” would denote that ring 2 was the thiophene. Thus, if you
were to name lanicemine with this scheme, it would be Apheni-2-pyridine. This
produces structures whose names can be a mouthful, but I fail to think of a
better solution. Nevertheless, this nomenclatural scheme should make it
relatively easy for the name of novel compounds to reflect their structure.
Some other Diarylethylamines:
So far, the diarylethylamines that have been shown to
have dissociative hallucinogenic effects in humans are, as mentioned before,
Ephenidine, Diphenidine, Methoxphenidine, Fluorolintane, and Isopropylphenidine.
Opioid Compounds
To illustrate how complicated designing diarylethylamines can be, how rife with pitfalls and exceptions it is, let’s look at some other compounds that technically fall within the diarylethylamine family but have quite different activity. Compounds with a piperazine as the amine have proven to be opioids (namely MT-45, AD-1211, and Diphenpipenol) though this may also be virtue of the various structures then appended to the other nitrogen in the ring, rendering it tertiary, or it may also be a function of variously placed hydroxy substitutions. It’s hard to pin down what exactly dictates that activity but something keeps them from being more selective regular NMDA antagonists. Perhaps a piperazine as the amine in an arylcyclohexylamine would also generate an opioid?
Lefetamine
Lefetamine was mentioned before as the representative diarylethylamine in medical literature for much of the 20th century. It was commercially available in postwar Japan where it was marketed a painkiller, then later saw abuse in Europe, but seems to have disappeared entirely since then [4]. It was sold under the trade name Santonel. Users reported a combination of opioid-like and stimulant effects. One study confirmed that it was at least in part, a µ-opioid agonist [4]. It is not yet known what activity it derives it stimulant properties from. It has not yet been explicitly tested for NMDA antagonist activity, though it’s entirely possible that it possesses this property.
One possibility lies in the fact that only the
(R)-enantiomer of lefetamine has been produced and tested in vivo, yielding
measurable opioid effects. When we look at dissociative diarylethylamines, such
as diphenidine, it is the (S)-enantiomer that possesses NMDA antagonist
properties, as opposed to the (R)-enantiomer, which shows lower affinity [1].
With this in mind, it is entirely possible that a racemic mixture of lefetamine
or simply a sample of the pure (S)-enantiomer may have different selectivity,
and could perhaps even have a high affinity for the NMDA receptor! This also
raises the questions of how a tertiary amine on a diarylethylamine behaves- it
appears for now that non-cyclic tertiary amines see reduced NMDA antagonist
activity- perhaps affinity increases with bulkier alkanes? Or perhaps some sort
of ring structure is necessary, as seen in Diphenidine. But for now, this is
pure conjecture, and lefetamine remains an interesting footnote in the history
of this family of chemicals.
 |
| "Ephenidine-2" |
Also worth mentioning is the compound Methylethylphenidine, aka N-methylephenidine, aka “Ephenidine-2”. As the name implies, it is a tertiary amine very similar in structure to lefetamine with a methylethyl tertiary amine. A single report exists for this substance, citing no dissociative effects, but rather unremarkable stimulant effects with a series of oral and intranasal doses ranging from 20-100 mg [20]. This supports the hypothesis that a noncyclic tertiary amine is likely to be show affinity for other receptors either alongside the NMDA receptor, or entirely instead of it.
Lanicemine and Remacemide
 |
| The structures of Lanicemine and Remacemide |
These are two other Diarylethylamines that were
produced and studied for possible medical uses. Though fairly different in
structure, they both share the property of being weak NMDA antagonists.
Lanicemine,also called AR-R15896, consists of a
primary ethylamine, where ring 1 is replaced with a 2-pyridine. It was
developed to be an antidepressant- possibly due to having a similar action to
ketamine but less ketamine-like dissociative effects [17]. Like many NMDA
antagonists, it was also studied as an anti-ischemic [8]. This is owed to a
phenomenon called trapping, where it is conjectured that certain molecules such
as PCP or MK-801 become “trapped” in the channel of the NMDA receptor,
amplifying their hallucinogenic effects (to put it extremely simply) [6]. This
is an example of what may happen when one of the typical phenyl rings is
substituted for another aromatic ring. Mealing et al. in the study “Structural modifications to an
N-methyl-D-aspartate receptor antagonist result in large differences
in trapping block” touches upon a number of diarylethylamines with other
aromatic rings replacing a phenyl [6]. Many of these compounds show higher
trapping than lanicemine, which hypothetically may suggest that they will
display certain familiar hallucinatory NMDA antagonist effects. The correlation
between trapping and activity as a dissociative isn’t direct, confirmed, or
fully understood however, and this is just conjecture.
Remacemide meanwhile, sports a methyl group sharing
the α-carbon with the phenyl, and an acetamide as the amine (on a secondary
amine). Remacemide has also been demonstrated to be a weak NMDA antagonist and
has been investigated thoroughly for the suite of possible medical applications
of NMDA antagonists (Anticonvulsants, antiparkinsonians, anti-ischemics etc.)
[9] [10]. While it is an NMDA antagonist it is noted for not producing familiar
NMDA antagonist “side effects”. Whether this is a result of the α-methyl group
or the acetamide is unknown, though it has been demonstrated that
diarylethylamines with α-methyl groups on a quaternary carbon shared with one
of the aromatic rings can retain affinity for the NMDA receptor (at least in
vitro) [1].
A-D2PV
 |
| Chemical Structure of A-D2PV (or α-d2PV) |
So its apparent that some relatively tame and
expectable modifications of the diarylethylamine backbone can yield an
unpredictable variety of receptor activities, some could possibly be
nonselective NMDA antagonists in addition to hitting other targets while others
may be selective for different receptor systems entirely! Only by developing
and testing more of them can we try and figure out what would yield a possible
interesting, nontoxic, and active substance that is sufficiently potent. There’s
a whole world to explore! Unlike the last essay, I am not including a section of what I think should be investigated next- simply put, we only have 3 dissociative compounds with enough data to provide a reference for their effects in vivo, it's hard to discern any clear patterns or development leads on that, beyond extending a secondary alkane chain and strapping substitutions to amines that are known to be active. Really any potential pathway within this family is worth investigating in my opinion. This is out of my hands now, I don't know anything about synth or orgo reactions. I hope this infomation will be valuable to an intrepid explorer.
Sources and Further Reading:
[1]- Berger ML, Schweifer A, Rebernik P, Hammerschmidt
F (2009) NMDA receptor affinities of 1,2-diphenylethylamine and
1-(1,2-diphenylethyl)piperidine enantiomers and of related compounds. Bioorg
Med Chem 17:3456–3462
[2]- Gray NM, Cheng BK (1989) 1,2-Diarylethylamines
for treatment of neurotoxic injury. Patent No. EP346791A1. G.D. Searle and Co.,
Chicago
[3]- Kang H, Park P, Bortolotto ZA, Brandt SD,
Colestock T, Wallach J, Collingridge GL, Lodge D (2017) Ephenidine: A new
psychoactive agent with ketamine-like NMDA receptor antagonist properties. Neuropharmacology.
112(Pt A):144-149
[4]- Mannelli P, Janiri L, De Marinis M, Tempesta E
(1989) Lefetamine: new abuse of an old drug--clinical evaluation of opioid
activity. Drug Alcohol Depend 24(2):95-101
[5]- Massa S, Stefancich G, Artico M, Corelli F,
Silvestri R, Pantaleoni GC, Fanini D, Palumbo G, Giorgi R (1989) Synthesis,
neuropsychopharmacological effects and analgesic-antiinflammatory activities of
pyrrole analogues of lefetamine. Farmaco 44(9):763-77
[6]- Mealing GA,
Lanthorn TH, Small DL, Murray RJ, Mattes KC, Comas TM, Morley P (2001)
Structural modifications to an N-methyl-D-aspartate receptor antagonist result
in large differences in trapping block. J Pharmacol Exp
Ther. 297(3):906-14
[7]- Morris H, Wallach J. From PCP to MXE: a
comprehensive review of the non-medical use of dissociative drugs. (2014) Drug
Test Anal. 6(7-8):614-632.
[8]- Palmer GC, Cregan EF, Bialobok P, Sydserff SG,
Hudzik TJ, McCarthy DJ .(1999) The low-affinity, use-dependent NMDA receptor
antagonist AR-R 15896AR. An update of progress in stroke. Ann N Y Acad Sci
890:406-20
[9]- Palmer GC, Murray RJ, Wilson TC, Eisman MS, Ray
RK, Griffith RC, Napier JJ, Fedorchuk M, Stagnitto ML, Garske GE (1992)
Biological profile of the metabolites and potential metabolites of the
anticonvulsant remacemide. Epilepsy Res. 12(1):9-20
[10]- Santangeli S, Sills GJ, Thompson GG, Brodie MJ
(2002) Na(+) channel effects of remacemide and desglycinyl-remacemide in rat
cortical synaptosomes. Eur J Pharmacol. 438(1-2):63-8
[11]- Tainter ML, Luduena FP, Lackey RW, Neuru EN
(1943) Actions of a series of Diphenyl-ethylamines. Journal of Pharmacology
and Experimental Therapeutics 77(4):317-323
[12]- Wallach J, Brandt SD (2018)
1,2-Diarylethylamine- and Ketamine-Based New Psychoactive Substances. Handb
Exp Pharmacol. 252:305-352
[13]- Wallach J, Colestock T, Agramunt J, Claydon MDB,
Dybek M, Filemban N, Chatha M, Halberstadt AL, Brandt SD, Lodge D, Bortolotto
ZA, Adejare A (2019) Pharmacological characterizations of the 'legal high'
fluorolintane and isomers. European Journal of Pharmacology 857:172427
[14]- Wallach J, Kang H, Colestock T, Morris H,
Bortolotto ZA, Collingridge GL, Lodge D, Halberstadt AL, Brandt SD, Adejare A
(2016) Pharmacological Investigations of the Dissociative 'Legal Highs'
Diphenidine, Methoxphenidine and Analogues. PLoS One. 11(6):e0157021
[15]-Wallach J, Kavanagh PV, McLaughlin G, Morris N,
Power JD, Elliott SP, Mercier MS, Lodge D, Morris H, Dempster NM, Brandt SD (2015)
Preparation and characterization of the 'research chemical' diphenidine, its
pyrrolidine analogue, and their 2,2-diphenylethyl isomers. Drug Test Anal 7(5):358-67
[16]- Wink CS, Meyer GM, Wissenbach DK, Jacobsen-Bauer A, Meyer MR,
Maurer HH (2014) Lefetamine-derived designer drugs
N-ethyl-1,2-diphenylethylamine (NEDPA) and N-iso-propyl-1,2-diphenylethylamine
(NPDPA): metabolism and detectability in rat urine using GC-MS, LC-MSn and
LC-HR-MS/MS. Drug Test Anal. (10):1038-48
[17]-
Zarate CA Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky
L, Brutsche NE, Smith MA, Luckenbaugh DA (2013) A randomized trial of a
low-trapping nonselective N-methyl-D-aspartate channel blocker in major
depression. Biol Psychiatry 74(4):257-64
[19]- https://www.bluelight.org/xf/threads/lefetamine.275135/
[20]- https://www.bluelight.org/xf/threads/the-small-handy-n-methyl-ephenidine-ephenidine-2-thread.781268/
[22]- Elliott SP, Brandt SD, Wallach J, Morris H,
Kavanagh PV (2015). First reported fatalities associated with the 'research
chemical' 2-methoxydiphenidine. J Anal Toxicol. 39(4):287-93
[23]- https://psychonautwiki.org/wiki/Diphenidine
[25]- https://www.reddit.com/r/researchchemicals/comments/jah4cn/ad2pv_any_new_user_reports/
[26]- https://www.reddit.com/r/researchchemicals/comments/ibebx5/ad2pv_12diphenyl2pyrrolidin1ylethan1one/
[27]- https://www.reddit.com/r/researchchemicals/comments/iq4i1a/ad2pv_is_anyone_into_it/